What we mean by compliant AI (and why it matters for pharma teams)
At VISFO, we’re not a group of AI obsessives chasing the next shiny thing. We’re scientists, strategists, and technologists who’ve spent years working in the pharmaceutical trenches. Many of us have sat where you sit now, faced with important questions and too little time to answer them. We know that decisions in Medical Affairs, Market Access, and Clinical Development don’t just need data. They need confidence. That’s why we’ve taken a different approach to AI, one that puts scientific integrity and compliance first.
We call it compliant AI, and it isn’t a catchphrase. It’s a set of principles designed to make sure the answers you get from HelixAI are not only fast, but trustworthy, defensible, and grounded in reality.
Let’s break down what that means in practice.
The problem with most AI in pharma
It’s no surprise that pharma teams are excited about the potential of AI. The promise is compelling: instant answers, automated content, summarized insights, and the dream of getting more done in less time. On paper, it looks like a silver bullet for overloaded teams juggling publication monitoring, stakeholder mapping, strategy alignment, and internal reporting.
But there’s a catch. And it’s a big one.
Most AI models weren’t built for this environment. They were trained on the open internet: blogs, Reddit threads, news articles, and Wikipedia entries. While that makes them excellent at forming sentences, it doesn’t mean they understand regulatory nuance, scientific standards, or the real-world implications of a misstep.
Let’s take a common scenario: a Medical Affairs team asks an AI assistant to summarize the most recent research on immune-related adverse events in checkpoint inhibitor therapies. If the AI isn’t grounded in evidence, it may generate a summary that sounds convincing but can’t be traced to any publication. If challenged, there’s no citation to review. If used in a medical slide deck or stakeholder discussion, that “summary” could lead to reputational or even regulatory risk.
Or consider a Market Access team crafting a payer value story. An AI model might generate a perfectly formatted HTA-style justification using outdated or irrelevant references. Without awareness of the specific jurisdictional context, or even basic source validation, the result might seem useful at first glance but be unfit for real use. That’s both inefficient and dangerous.
Without strong evidence foundations and clear attribution, AI actively creates problems.
This is the fundamental problem with most off-the-shelf AI in pharma. It gives you output, but not certainty. Answers, but not trust. And in a high-stakes, highly regulated environment like ours, trust is everything.
That’s why VISFO’s approach starts from the opposite direction, not by generating content, but by structuring the right data and ensuring every insight is traceable to a credible, auditable source. Whether you’re reviewing trial endpoints, comparing pricing benchmarks, or briefing a Field Medical team, there’s one rule that always applies: if you can’t trust the output, you can’t use it. So we made that rule our baseline.
Grounded in the evidence
One of the first decisions we made was that HelixAI would never just hallucinate an answer. Everything it generates is based on real, accessible, attributable evidence.
This is what we call grounded AI. Rather than giving you a slick response that might sound smart but can’t be traced back to a reliable source, HelixAI surfaces the actual evidence and then helps you make sense of it.
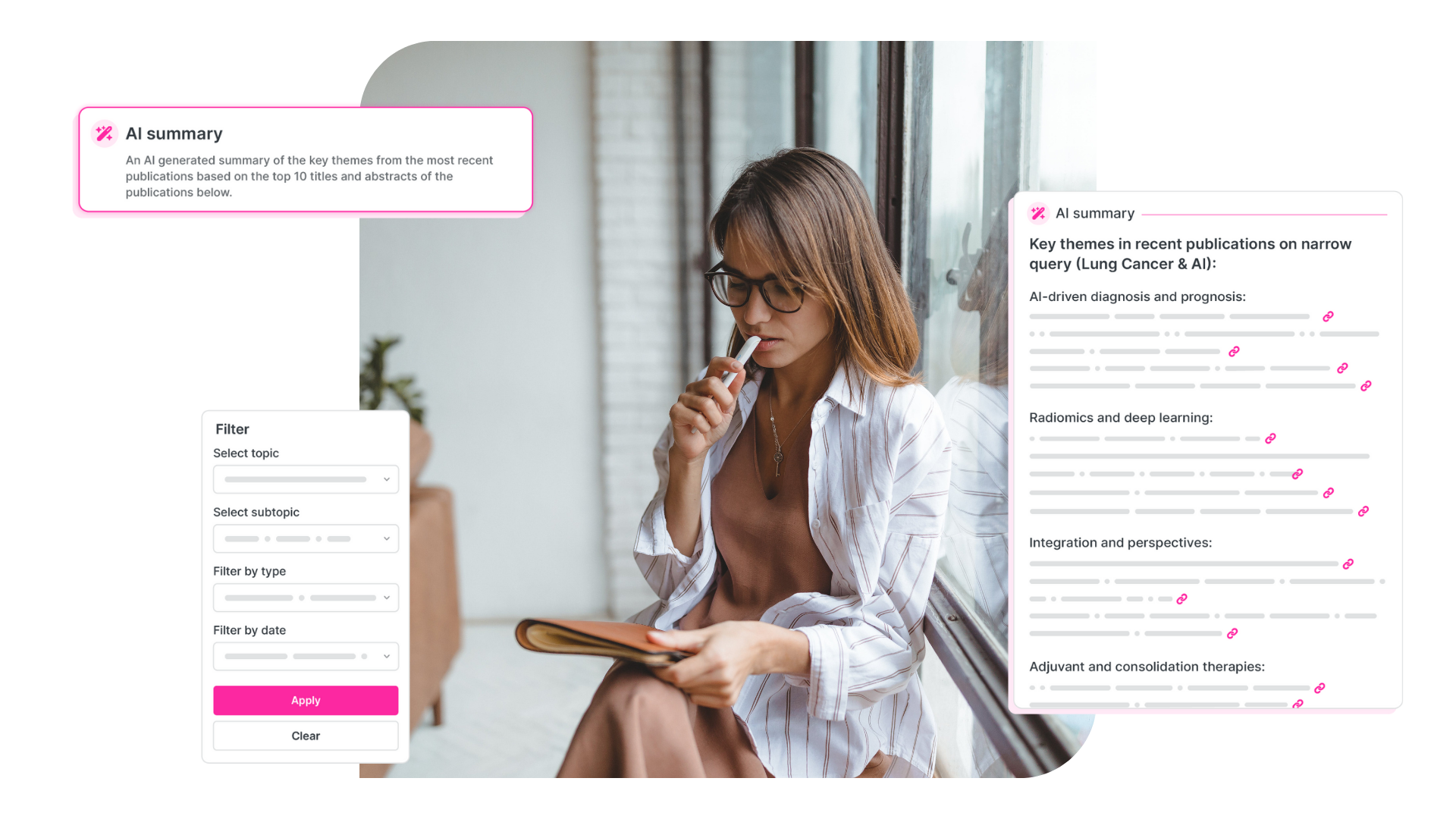
For example, if you’re a Medical Affairs lead looking to understand unmet need in a subpopulation of non-small cell lung cancer (NSCLC) patients, HelixAI won’t just summarize what it thinks that means. It will pull the top 10 most recent publications based on relevance, rank them using our proprietary impact scoring, summarize their contents, and give you links back to each PubMed record.
The result is confidence. You’re not taking AI’s word for it. You’re using it to accelerate your review of the literature, while maintaining the quality and rigour that’s expected in your role.
Everything attributed, nothing left to chance
One of the biggest complaints about general AI systems in pharma is that they’re black boxes. You get a result, but you don’t know where it came from - which, in this industry, is a non-starter.
That’s why HelixAI attaches source references to everything. Whether it’s a strategic summary of the latest literature on immune checkpoint inhibitors or a visualization of expert networks in autoimmune disease, every data point can be traced.
In practical terms, this means:
- Every AI-generated summary is footnoted with its source papers,
- Every KOL profile shows you the exact publications contributing to their ranking,
- Every strategic insight is built on verifiable data, not just opinions or assumptions.
You can check the evidence, validate the interpretation, and export what you need for a team review or submission. Nothing is hidden. Nothing is made up.
Scientific ranking, not just search
Most systems give you access to data. HelixAI goes a step further and helps you prioritise it.
Our disambiguation and dynamic impact scoring engine means that publications and authors are ranked differently depending on your specific context. For example:
- A Market Access user looking at treatment costs in France will see different authors and literature than a Medical Affairs team exploring immunotherapy side effects in the US
- An Early Asset team researching a niche mechanism of action (MoA) in atopic dermatitis will see subtopic-relevant insights, not just a list of generic dermatology papers
This context-aware ranking is made possible by years of work disambiguating authorship, standardising data inputs, and creating modular filters that reflect how strategic questions are actually asked inside pharma companies.
We’re not searching the literature, we’re understanding it in the same way you need to.
Built on an ontology that speaks your language
Behind the scenes, HelixAI uses an expert-built ontology to structure how the AI understands the data. A framework that reflects how pharma professionals think about problems.
For instance, we’ve mapped:
- The full landscape of pricing and reimbursement topics, from willingness to pay to comparator pricing,
- The different dimensions of clinical evidence, from biomarker stratification to endpoint alignment,
- Stakeholder types and their strategic relevance, such as payer-influencing KOLs, trial investigators, or digital opinion leaders.
Because of this, when a user asks HelixAI a strategic question like “Which publications support price justification for biosimilars in Europe?”, the system knows how to unpack that and serve up structured insights, summaries, and references in a format that’s actually useful.
Compliance is the foundation, not a feature
HelixAI wasn’t built to impress people with flashy AI demos (even though it does). It was built to support real teams working in complex, high-stakes environments.
That’s why compliance isn’t something we’ve added on top. It’s baked into the foundation. From how we ingest data, to how we structure it, to how we ensure that what comes out the other side is both useful and defensible.
This isn’t just about saving time or looking smart in a meeting. It’s about doing work that stands up to scrutiny, that respects the science, and that helps teams do what they do best: making informed decisions that improve patient lives.
If that sounds like something worth exploring, you can learn more about HelixAI on our dedicated product page.

